Abstract
Purpose of Review
A variety of neurological complications have been reported following the widespread use of the COVID-19 vaccines which may lead to vaccine hesitancy and serve as a major barrier to the public health aim of achieving protective herd immunity by vaccination. In this article, we review the available evidence regarding these neurological adverse events reported, to provide clarity regarding the same so that unfounded fears maybe put to rest.
Recent Findings
There is a greater than expected occurrence of severe neurological adverse events such as cortical sinus venous thrombosis, Bell’s palsy, transverse myelitis, and Guillain–Barré syndromes along with other common effects such as headaches following different kinds of COVID-19 vaccination. Precipitation of new onset demyelinating brain lesions with or without detection of specific antibodies and worsening of pre-existing neurological disorders (like epilepsy, multiple sclerosis) are also a matter of great concern though no conclusive evidence implicating the vaccines is available as of now.
Summary
The COVID-19 pandemic is far from being over. Till such time that a truly effective anti-viral drug is discovered, or an appropriate therapeutic strategy is developed, COVID-appropriate behavior and highly effective mass vaccination remain the only weapons in our armamentarium to fight this deadly disease. As often occurs with most therapeutic means for the treatment and prevention of any disease, vaccination against COVID-19 has its hazards. These range from the most trivial ones like fever, local pain and myalgias to several potentially serious cardiac and neurological complications. The latter group includes conditions like cerebral venous thrombosis (curiously often with thrombocytopenia), transverse myelitis and acute inflammatory demyelinating polyneuropathy amongst others. Fortunately, the number of reported patients with any of these serious complications is far too low for the total number of people vaccinated. Hence, the current evidence suggests that the benefits of vaccination far outweigh the risk of these events in majority of the patients. As of now, available evidence also does not recommend withholding vaccination in patients with pre-existing neurological disorders like epilepsy and MS, though adenoviral vaccines should be avoided in those with history of thrombotic events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunization has long been the foundation of public health policy and has rendered various deadly diseases which once felled millions into the “rare,” “eradicated,” and “easily treatable” categories [1]. The World Health Organization (WHO) estimates that 2–3 million lives are saved each year by the current national immunization programs [2]. When humanity was first faced with the SARS-CoV-2 virus in the winter of 2019, there followed an initial period of “trial and error” method of treating the virus and its myriad manifestations. Work also progressed at breakneck speed for the identification of the genomic sequence of the virus and then to develop a vaccine against it. Scientists and researchers were successful like never before in coming up with several effective vaccines against the deadly virus in record time, leading to an upsurge of hope around the world that this devastating pandemic would finally be over. However, like every other human creation, these vaccines proved to be a double-edged sword. Soon after the introduction of the widespread vaccine drives, several complications (some substantiated, some not) began to be reported. The neurological system seemed to be one of the most affected by these vaccines and is the subject of our discourse here [3, 4].
Principles of Vaccination
The fundamental concept of vaccination rests on the principle of “immune memory” [5]. A vaccine can be defined as a biological/biochemical product that can be administered to “safely” produce an immune response that confers “protection” against infection and/or disease on subsequent exposure to a pathogen. The term “protection” refers to a state in which an individual will not develop a disease after being challenged with a pathogen [5]. A vaccine contains “antigens” that are either derived from the pathogen or produced synthetically to represent components of the same. These antigens are capable of inducing an immune response in the host that the immune system will then remember, and therefore protect the host against subsequent exposures to these pathogens. This adaptive immune response is mediated by B cells which produce antibodies (humoral immunity) and by T cells (cellular immunity). All vaccines in routine use confer protection through the induction of antibodies [5]. These antigens are usually various protein components of the pathogen, but rarely polysaccharide vaccines have also been used against bacterial infections. Efficacy of a vaccine is measured in terms of the protection conferred by it and can be estimated in clinical trials endpoints such as prevention of infection, a reduction in disease severity or a decreased rate of hospitalization and /or death.
COVID Vaccines and Their Mechanisms of Action
COVID-19 vaccines prime the immune system to create antibodies against SARS-CoV-2. The vaccines use a structure similar to spike (S) protein, which is present on the surface of SARS-CoV-2 and is used for viral endocytosis to the host cells. The COVID-19 vaccines include messenger RNA (mRNA), vector, protein subunit, and inactivated/weakened whole virus vaccines [6•, 7•, 8•, 9•, 10•, 11•, 12•, 13•]. Engineered mRNA COVID-19 vaccines namely Pfizer-BioNTech (BNT162b2) and the Moderna (mRNA-1273) give the cells instructions to make S protein which then leads to the generation of protective antibodies. After delivering instructions, the injected mRNA breaks down and does not enter the nucleus of the host cells [6•, 7•]. In vector COVID-19 vaccines which include Ad26.COV2.S [8•], Sputnik V (rAd26-S and rAd5-S) [9•], and ChAdOx1 nCoV-19 SARS-COV-2 (AstraZeneca/Covishield) [10•], genetic material is inserted in a viral vector. The vector delivers the genetic material to the host cells that make copies of the S protein on their surfaces. The immune system then responds by creating antibodies and defensive mononuclear white blood cells like lymphocytes and plasma cells. Protein subunit COVID-19 vaccines such as Novavax (NVX-CoV2373) include only harmless S protein which stimulates the immune system [11•]. Inactivated or weakened COVID-19 vaccines do not cause disease, but still, stimulate the immune system, such as the Covaxin developed by Bharat Biotech and the Indian Council of Medical Research, and the CoronaVac and Sinopharm developed in China [12•, 13•, 14•]. The very recently introduced bivalent COVID-19 vaccines include a component of the original virus strain to provide broad protection against COVID-19 and a component of the omicron variant to provide better protection against COVID-19 caused by the omicron variant. The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least 2 months after completing primary or booster vaccination in subjects above the age of 12 years.
Non-neurological Complications of COVID-19 Vaccines
The most common non-neurological adverse events following COVID-19 vaccination include fever, chills and rigor, fatigue, muscle and joint pains, nausea, diarrhea, and rashes. Local site reactions including pain and tenderness, local warmth, pruritus, bruising, and axillary lymphadenopathy have also been commonly reported. Local and systemic allergic reactions may occur [15••, 16]. Pillay and colleagues conducted a systematic review of myocarditis and pericarditis after COVID-19 mRNA vaccination. The relative incidence of myocarditis was highest among young males between 12 and 29 years of age after a second dose, and with Moderna’s mRNA vaccine than after Pfizer-BioNTech’s vaccine. The risk of myocarditis/pericarditis is lower when the second dose is administered after 30 days of the first dose. Finally, the study concluded that most patients had a good outcome and mortality was low after these cardiac complications. The occurrence of myocarditis overall is rare after vaccination [17•].
Neurological Complications of COVID-19 Vaccines
Neurological adverse events following COVID-19 vaccination are generally mild and transient [18••]. The most commonly reported side effects are headache, fatigue, muscle, and joint pains. As with any other injectable vaccine, local injection site reactions such as swelling, redness, and pain are also well known. These mild neurological effects are seen with all kinds of COVID-19 vaccines.
Incidence of Neurological Adverse Events
In a Mexican study, 6536 adverse events were recorded after Pfizer-BioNTech vaccination among 704,003 subjects. Sixty-five percent of these had at least one neurologic complication, 99% were mild and transient. The most commonly reported events included headache, transient sensory symptoms, and generalized weakness. The only severe adverse events recorded were seizures in 7, Guillain-Barré syndrome in 3 patients, and transverse myelitis in 2 patients [19••]. Similar rates were seen in a South Korean study by Kim et al. looking at adverse effects after the first dose of ChAdOx1 nCoV-19 and BNT162b2 vaccines [20]. In another study conducted amongst healthcare workers in the UK after two doses of the BNT162b2 mRNA vaccine, the most common adverse effects were myalgia, headache, chills, fatigue, and fever. These complications were higher after the second dose than with the first dose [21].
Classification of Neurological Adverse Effects of COVID Vaccines
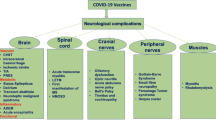
There are two ways in which one may classify the neurological side effects of these COVID vaccines; one is by classifying these as those affecting the central nervous system and those affecting the peripheral nervous system. Another way of classifying these maybe those that are considered serious/severe and those which are minor. The former classification scheme is depicted in Fig. 1. Below, we elaborate on individual neurological complications reported after vaccination.
Neurological Adverse Events
Headache is the most frequent mild neurological complication reported and occurs with all of the approved COVID vaccines. This usually starts within the first few hours after the vaccination and in most cases, resolves spontaneously within the next 48 h. The location is usually bifrontal or temporal, dull aching in nature with varying intensity. Frequent accompaniments include fever, chills, myalgia, and fatigue [22]. However, there is another type of headache which is far more severe and needs to be distinguished from the first type. This occurs about a week after the vaccine and is secondary to more ominous neurological complications like cortical venous thrombosis, intracerebral, or subarachnoid hemorrhage [23•].
Severe Neurological Adverse Events
Severe or serious adverse reaction following immunization is defined as a post-vaccination event that is either life-threatening, requires hospitalization, or results in severe disability. The WHO lists Guillain-Barré syndrome, seizures, syncope, encephalitis, Bell’s palsy, and strokes as serious neurologic adverse events [24]. These may be the source of vaccine hesitancy, and it is important to report and analyze these events to better understand whether a clear-cut relationship between the two actually exists.
Cerebral Vascular Events
The whole range of cerebral vascular events from arterial strokes and venous thromboses to intracerebral and subarachnoid hemorrhages have been reported, predominantly with the adenoviral vector vaccines, often associated with severe vaccine-induced thrombotic thrombocytopenia (VITT), which manifests 5 to 30 days after the vaccination. The clinical picture is similar to that of heparin-induced thrombocytopenia [25••].
Cerebral Venous Sinus Thrombosis
Cerebral venous sinus thrombosis (CSVT) is the most feared COVID vaccine-associated neurological complication. This should be suspected in all patients who develop a persistent headache post-vaccination, which is unresponsive to analgesics and often associated with a dip in sensorium and/or seizures and focal deficits. As mentioned earlier, CSVT is commonly associated with VITT.
Mechanism
VITT is caused by antibodies that recognize platelet factor 4 (PF4) bound to platelets. The antibodies are immunoglobulin G (IgG) molecules that activate platelets via low-affinity platelet FcγIIa receptors causing platelet aggregation and activation, leading to hypercoagulability, but with decreased platelet count. A state of thrombotic thrombocytopenia ensues resulting in venous thrombosis with marked elevation of D-dimer due to increased generation of fibrin degradation products. Microscopic findings show inflammatory vascular thrombotic occlusions occurring in the vessels of multiple body organs [26••]. The vector-based vaccines contain genetic material of SARS-COV-2 that is capable of encoding the spike glycoprotein. Possibly leaked genetic material binds to platelet factor 4 that subsequently leads to the formation of autoantibodies [27••, 28].
Post-vaccination CSVT has a propensity for younger females [23•, 25••, 26••, 27••, 28,29,30,31,32,33,34, 35••, 36••, 37, 38]. The commonly implicated vaccines in CSVT have been the Astra Zeneca and Johnson and Johnson vaccines [26••]. Scully et al. published a case series of 21 patients who had venous sinus thrombosis with documented thrombocytopenia, which developed 6 to 24 days after the first dose of the viral vector-based vaccines [27••]. Presence of autoantibodies against platelet factor 4 and elevated D-dimer levels was also demonstrated.
Tiede reported five cases of prothrombotic immune thrombocytopenia after vaccination with viral vector-based vaccine (Vaxzevria) in Germany which clinically manifested as CSVT, splanchnic vein thrombosis, arterial cerebral thromboembolism, and thrombotic microangiopathy within 2 weeks of vaccination [31].
Pottegård et al. assessed the rates of thrombotic events in the first 28 days after vaccination with the Oxford-AstraZeneca vaccine ChAdOx1-S in Denmark in 148,792 people and 132,472 people in Norway and compared them with rates observed in the general populations to determine the excess risk, if any. Authors estimated an increased rate for venous thromboembolism corresponding to 11 excess events per 100,000 vaccinations with 2.5 times excess cerebral venous thrombosis events per 100,000 vaccinations [35••].
Krzywicki et al. analyzed data of 213 cases with post-vaccination CSVT, of which 187 occurred after adenoviral vector vaccines and 26 after mRNA vaccines; thrombocytopenia was only reported with adenoviral vaccines. CSVT after adenoviral vaccines also carried poorer prognosis; 38% patients in adenoviral vaccine group died, while 20% succumbed in the mRNA vaccine group [36••]. Perry et al. analyzed 95 cases of vaccine associated CSVT, 70 of which were associated with VITT [37]. Patients with VITT were younger, had more thrombosed intracranial veins and poorer outcomes. Use of non-heparin anticoagulants and IVIg was associated with better outcomes [38].
When diagnosing CSVT related to COVID-19 vaccination, a temporal relationship must be established (within 30 days of vaccination) and other prothrombotic states must be excluded. However, whether the vaccines have a role in precipitating the thrombosis in susceptible individuals with pre-existing prothrombic states is not established [39•] (Fig. 2). Such patients should also be checked for fresh COVID-19 infection by RT-PCR as CSVT may also occur in infected cases.
A, B MR venography in a 60-year-old lady with prior history of migraine, presenting with a three week history of increasing headaches starting about a fortnight after her second dose of AstraZeneca (COVISHIELD vaccine), showing segmental partial occlusion of both transverse sinuses. She did not have any thrombocytopenia or raised D-dimer but had a low protein S level suggesting a pre-existing prothrombotic state. She was started on apixaban 2.5 mg twice daily. Her headaches were relieved. Repeat venography done over 6 months later revealed recanalization of the thrombosed segments (C). Her protein S level was persistently low. She was advised to continue apixaban indefinitely. She had no problem after her third booster dose of the same vaccine (adapted from [39•], with permission)
National Institute for Health and Care Excellence (NICE) guidelines recommend treatment of patients with VITT and thrombosis with intravenous immunoglobulin, at a dose of 1 g/kg which may be repeated if there is further clinical deterioration. In patients with insufficient response, methylprednisolone 1 g intravenously for 3 days or dexamethasone 20 to 40 mg for 4 days can be used [40••]. Heparin should be avoided, and alternative anticoagulants like argatroban, bivalirudin, fondaparinux, rivaroxaban, or apixaban should be used [36••, 37, 38]. Patients with very low platelet count should be treated either with argatroban alone or a combination of argatroban and platelet transfusion [41].
Arterial Ischemic Stroke
Ischemic stroke following COVID-19 vaccination are also reported though rarely, usually seen in the context of VITT [42•]. Pottegard et al. described an increased rate of venous thromboembolic events but not of arterial events among recipients of ChAdOx1 nCoV-19 [35••]. Blauenfeldt et al. described a female who presented with bilateral adrenal necrosis, 7 days after receiving the adenoviral (ChAdOx1) vaccine. She subsequently developed a malignant right cerebral infarction, secondary to occlusion of the right internal carotid artery leading to her death. She had thrombocytopenia, elevated D-dimer, and PF-4 antibodies in her blood [43].
De Michele et al. described two fatal cases with VITT-associated malignant MCA infarcts one of which was treated with mechanical thrombectomy, with additional portal and pulmonary vein thrombosis [44]. Al-Mayhani et al. described three cases of VITT presenting with arterial strokes [45]. Authors opined that young patients with arterial stroke after receiving the COVID-19 vaccine should always be evaluated for vaccine-induced thrombotic thrombocytopenia.
Question may be raised whether vaccine-eligible subjects need prior screening for any coagulopathy before vaccination which would be very expensive. Duration of oral anticoagulation therapy may be 6 months for subjects without pre-existing prothrombotic states, in whom treatment should be continued lifelong.
Stroke Mimics and COVID-19 Vaccination
A recent report from Thailand highlights a large number of cases with a distinctive focal neurological syndrome among those receiving the Chinese vaccine CoronaVac but not with the AstraZeneca vaccine [46•]. This develops within the first few days of the first dose of vaccination and consists of transient hemisensory or hemimotor disturbances, at times associated with visual phenomenon developing in the corresponding hemifield of vision. Hemicranial or holocranial headaches are often present. Diffusion-weighted MRI brain and MR angiography were normal in all patients. SPECT studies however demonstrated hypoperfusion in the contralateral hemisphere during the acute phase followed by hyperperfusion with clearing of symptoms. This has been likened to the phenomenon of “cortical spreading depression” occurring in subjects with migraine with aura. Postulations regarding the triggers for this include precipitation by vaccination-related stress and an immune response to the vaccine.
ICH
Both intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) can occur after COVID-19 vaccination, which can be primary or secondary to venous thrombosis [26••, 27••, 47,48,49]. While ICH after COVID-19 vaccination can occur in the context of VITT, de Melo Silva et al. described a primary hemorrhagic stroke following ChAdOx1nCoV-19 vaccination in a patient without thrombocytopenia, or coagulopathy [48]. Finsterer et al. suggested that the second dose of vaccination may be followed by ICH even when the first dose was uneventful [49]. Most of these cases were reported following ChAdOx1 nCoV-19 vaccine and in people 30 to 57 years of age, 5 to 12 days after the vaccination.
SCLS
This is a potentially life-threatening immune disorder characterized by transient, recurrent episodes of vascular endothelial hyperpermeability triggered by viral upper respiratory infections, and maybe seen after both adenoviral and mRNA COVID vaccines. Hypotension, hemo-concentration, hypoalbuminemia, and anasarca are prominent features. Treatment is aimed at correcting hypovolemia and avoiding end-organ damage; prophylactic monthly administration of immunoglobulins can prevent further episodes. Adenoviral vector vaccines against SARS-CoV-2 are not advised for patients with a history of systemic capillary leak syndrome (SCLS). Monoclonal gammopathy of uncertain significance is observed in 68–85% of patients with SCLS [50].
Encephalopathy
Acute encephalopathy clinically manifests either with delirium, decreased consciousness, and a fluctuating sensorium with impaired attention. Reports of acute encephalopathy have been reported following mRNA vaccines, especially the Moderna vaccine [51, 52•, 53]. Elderly patients are affected and present with decreased attention and confusion with diffuse slowing on electroencephalogram (EEG) and negative neuroimaging. EEG may also show triphasic waves or nonconvulsive status. Workup for other causes of delirium is negative. Steroid-responsive encephalopathy was also reported in a young male following vaccination by Ali-Mashdali with elevated CSF protein levels [53].
ADEM
This is an acute inflammatory demyelinating disorder of the central nervous system which is associated with recent infections and vaccinations. Case reports of acute disseminated encephalomyelitis (ADEM) have been reported post-COVID vaccination with SinoVac and the Astra-Zeneca vaccines [54••, 55, 56]. Clinical manifestations include altered sensorium, seizures and focal neurological deficits. MRI showed multiple T2/FLAIR hyperintense lesions involving the periventricular and juxtacortical regions. Treatment is in the form of high-dose intravenous steroids and/or intravenous immunoglobulins. Most patients recovered well, though mortality had been reported.
Post‑vaccinal Encephalitis
Zuhorn et al. reported a series 3 patients, who presented with post-vaccinal encephalitis, occurring 7 to 11 days after administration of adenoviral ChAdOx1 nCov-19 vaccine. All patients fulfilled the diagnostic criteria for possible autoimmune encephalitis and presented with cognitive decline, seizures, and gait dysfunction; neuroimaging was non-contributory. CSF pleocytosis and good response to corticosteroids was noted in all [57••]. Presentations of brainstem encephalitis with diplopia and opsoclonus-myoclonus syndromes after COVID vaccination have also been reported, all with good response to steroid therapy [58].
Acute necrotizing encephalopathy (ANE) is a rare neurological disorder arising from a para- or post-infectious “cytokine storm.” Bensaidane et al. reported the case of a 56-year-old male who developed ANE 2 days following the first dose of ChAdOx1 nCoV-19 vaccination. MRI brain showed hyperintensities involving bilateral thalami, with diffusion restriction. The patient was treated with high-dose corticosteroids [59].
Transverse Myelitis
Acute transverse myelitis is a rare, acquired inflammatory spinal cord disorder manifesting with rapid onset weakness, sensory loss and bowel and bladder dysfunction. MRI demonstrates T2/FLAIR hyperintensity which is often holocord and maybe longitudinally extensive. Autoimmunity via mechanism of molecular mimicry is implicated. Adenoviral-based vaccines are more frequently associated with this presentation, though even inactivated virus vaccine and mRNA-based vaccines have been implicated [60, 61••, 62,63,64,65]. Both short and long segment myelitis have been reported after COVID vaccination; outcomes are favorable with high dose intravenous steroids.
During the phase III trial of Oxford/AstraZeneca vaccine, 2 patients developed transverse myelitis; one 14 days after the booster shot which was considered to be idiopathic. The second case was reported 68 days post-vaccination in a patient who was previously diagnosed with multiple sclerosis and was considered to be not associated with vaccination [63, 64]. Possibly, antigens present in the COVID-19 vaccine, or its adenovirus adjuvant induce an immunological reaction in the spinal cord leading to myelopathy. The occurrence of 3 reported acute transverse myelitis adverse events amongst 11,636 participants in the vaccine trials was considered high and a cause of concern [65]. Overall, the incidence of transverse myelitis after COVID vaccination remains higher than is expected in the general population and prompt diagnosis and treatment is needed to ensure good outcomes.
COVID-19 Vaccination and Pre-existing or New-Onset CNS Demyelinating Disorders
Quintanilla-Bordás et al. reported a case series of four patients that received mRNA COVID-19 vaccination while already suffering from symptoms related to CNS demyelination due to yet undiagnosed MS [66•]. All of these patients presented with exacerbation of ongoing symptoms after vaccination with MRI features suggestive of highly active MS, fulfilling McDonald 2017 criteria; high serum light-neurofilament levels and oligoclonal bands restricted to the CSF. It is recommended that patients experiencing acute neurological symptoms should seek medical attention, especially before vaccination.
On the other hand, Epstein et al. in a multicenter US study including 1164 participants, found no evidence of neuroimmunological disease worsening after vaccination [67••]. Dinoto et al. in another multicenter Italian study assessed the frequency of relapses after SARS-CoV-2 vaccination (Pfizer and Moderna) in patients with NMOSD and MOGAD. The frequency of relapses within one month of vaccination was 4% (1/26) in the AQP4-IgG + NMOSD group and 0 in the MOGAD group [68]. In these patients, the potential benefits of vaccination outweighed the risk of relapses. The jury is still out about the safety, at least of the mRNA vaccines, for patients with active relapsing–remitting MS, NMOSD and MOGAD. The need of the hour is large-scale real-world evidence for further validation.
Neuroleptic Malignant Syndrome
This is a life-threatening complication of many antipsychotic medications presenting with hyperpyrexia, altered sensorium, muscle rigidity, and autonomic dysfunction with markedly elevated creatine phosphokinase levels and subsequent renal dysfunction due to myoglobinuria. Various case reports have recorded the occurrence of this syndrome in elderly patients on antipsychotics who had recently received the COVID-19 vaccine, the real offender remaining uncertain [69, 70].
Seizures
There have been case reports of new-onset seizures in patients after COVID vaccination, where neuroimaging revealed no acute changes, no pre-existing brain lesions and workup for other causes of acute symptomatic seizures was negative. Seizure semiology may range from focal seizures with impaired awareness to bilateral tonic–clonic seizures and status epilepticus [71, 72, 73•]. A more important question is whether COVID-19 vaccination is safe in subjects with pre-existing epilepsy. Various studies have demonstrated that there is no significant effect of COVID vaccines on seizure frequency in patients with epilepsy. Such studies demonstrate that COVID-19 vaccines have a good safety and tolerability profile in the short term, in patients with epilepsy though there is high levels of vaccine hesitancy amongst these patients [74••, 75•, 76,77,78]. A UK survey noted that in most people with Dravet syndrome, SARS-CoV-2 vaccine does not appear to be associated with an increase in the frequency or duration of seizures, even in those who develop fever post-vaccination [79•]. Similarly, the safety of a Chinese virus inactivated vaccine (Sirolimus) in children with tuberous sclerosis (TS) who were being treated with the mTOR inhibitor rapamycin has also been demonstrated [80].
Bell’s Palsy
Several case reports have demonstrated the relationship between COVID-19 vaccination and Bell’s palsy, especially with the mRNA vaccines [81,82,83,84, 85••]. An article published in the Lancet analyzed the combined phase 3 data of Pfizer and Moderna trials and noted that the rate of Bell’s palsy was 3.5 to 7 times higher than expected in the general population [85••]. Facial palsy in most cases recovered with or without treatment. This being such a self-limited side effect, considerations about an elevated risk for Bell’s palsy should not lead to any vaccine hesitancy.
Olfactory Dysfunction
Olfactory dysfunction is the most frequent neurological complication of COVID-19 infection. Lechien et al. reported 6 cases of post-COVID vaccine olfactory and gustatory disorders in patients with negative nasal swab, 4 after the AstraZeneca vaccine and 2 after Pfizer vaccine, lasting from 4 to 42 days with no long-term side effects [86]. Keir reported a case in which the subject felt as if he was “smelling smoke” after the Pfizer vaccine; MRI brain showed olfactory bulbs and bilateral olfactory tract enhancement—the exact pathogenesis remaining unknown [87].
Abducens Nerve Palsy
Multiple case reports have reported a temporal relationship between COVID vaccination and isolated sixth cranial nerve palsies, with normal neuroimaging where no other causative factors were found, likely secondary to immune-mediated localized demyelination or vasculitis [88].
Ocular Manifestations
A variety of ocular manifestations have been reported post-vaccination, including lid edema, superior ophthalmic vein thrombosis, Tolosa-Hunt Syndrome, uveitis, choroiditis, retinal necrosis, macular retinopathy, central retinal vein occlusion, and optic neuritis (ON). Again, the incidence of these adverse events was so low that no definitive causal relationship could be attributed to the vaccines [89,90,91]. A multinational review of ON after vaccination found that most cases occurred after AstraZeneca vaccination with one-fourth showing myelin oligodendrogliocyte (MOG) antibody positivity, and visual outcomes were good, with or without treatment. The MOG antibody-positive patients showed contrast enhancement in the whole length of the optic nerve. This study also found that the incidence of post-vaccination ON was lower than the incidence of ON otherwise [92••].
Otologic Manifestations
A variety of otologic manifestations have been reported following COVID-19 vaccination, including new-onset, or worsening of pre-existing hearing loss, vertigo, and tinnitus. However, most authors concluded that there were no significant increases in otologic complications after COVID-19 vaccination as compared with the incidence in the general population [93].
GBS
Guillain‑Barré syndrome (GBS) is a rare but serious post-infectious immune-mediated polyradiculoneuropathy which manifests as a lower motor neuron type of areflexic sensorimotor non-length dependent quadriparesis. Significant medical literature exists which has established the link between COVID infection and GBS. Similarly, all kinds of COVID-19 vaccines have also been found to be associated with Guillain-Barré syndrome, though the incidence is higher with adenovirus-based vaccines [94, 95•, 96,97,98]. Post-vaccination GBS generally affects older adults within 2 weeks of vaccine administration. Clinical presentation is indistinguishable from GBS secondary to other causes. Nerve conduction studies show a demyelinating sensorimotor polyradiculoneuropathy pattern, and CSF examination shows albumino-cytological dissociation. Response to immunotherapy is generally good. The proposed pathogenesis is via autoantibody-mediated immunological damage of peripheral nerves and radicles through the mechanism of molecular mimicry between structural components of peripheral nerves and the vaccine components.
Small Fiber Neuropathy
A couple of case reports have demonstrated that small fiber neuropathy may be a rare complication of COVID-19 vaccination. Patients present with a severe burning sensation involving the extremities with normal electrodiagnostic studies [99].
Parsonage‑Turner Syndrome
Parsonage-Turner syndrome or neuralgic amyotrophy usually manifests clinically with acute onset unilateral shoulder pain with progressive involvement of the brachial plexus and upper limb weakness. Some case reports have documented the occurrence of Parsonage-Turner syndrome following COVID-19 vaccination [100].
Herpes Zoster
McMahon et al. reviewed cutaneous reactions to mRNA COVID-19 vaccines in 414 patients, of which 5 (1.9%) developed herpes zoster [101]. These vaccines possibly induce dysregulation of T-cell function, thereby allowing latent herpes zoster virus to reactivate.
Myositis and Rhabdomyolysis
Cases of fatal myositis with rhabdomyolysis and acute kidney injury necessitating renal replacement therapy have been reported post-COVID vaccination [102•, 103]. Kimura and colleagues described a patient who developed recurrent, relapsing muscle weakness after the Pfizer vaccine with MRI demonstrating myoedema.
Worsening of Pre-existing Neurological Disorders
Exacerbations of previous neurological diseases have been documented with COVID vaccinations. The cases of demyelinating disorders and epilepsy had already been discussed. Other disorders showing transient worsening following vaccination include myasthenia gravis, dementia and movement disorders such as Parkinsonism where temporary worsening of motor symptoms and severe dyskinesias have been reported. Even though medical literature is far from conclusive in these cases because of the low numbers, it is evident that the risks of COVID-19 infection far outweigh the transient exacerbation of these pre-existing disorders [104••, 105, 106, 107••, 108•].
Functional Neurological Disorders
Functional neurological disorders (FNDs) are those which produce varied neurological manifestations without any demonstrable abnormalities on extensive workup and are often triggered by physical or emotional stress. A variety of these FNDs have come to forefront after the COVID-19 vaccine drive; non-epileptic seizures characterized by paroxysms of bizarre hyperkinetic movements with no electrographic correlate, while others presented as variable limb weakness mimicking a cerebral vascular event [108•, 109,110,111, 112••, 113]. Ercoli and colleagues described a middle-aged man who immediately reported bilateral facial weakness which resolved in an hour after vaccination. The same gentleman after the second dose of vaccine, complained of respiratory distress and swollen tongue which resolved after receiving corticosteroids; however, he developed new symptoms in the form of right hemiparesis, and then facial sensory loss. A detailed workup of this patient failed to demonstrate any abnormality to explain the aforementioned varied manifestations and a diagnosis of functional neurological disorder was arrived at [113].
Concluding Remarks
Within a little over 2.5 years of the emergence of the novel SARS-CoV-2 virus infection that caused the worst pandemic the world had ever seen, the causative pathogen was determined, vaccine targets identified, mass scale vaccine manufacturing developed, large-scale phase 1 through phase 3 trials were conducted at multinational levels, data have been reported and finally millions of the world population have been vaccinated at least with one or two doses. This process demonstrates what is possible in the context of motivated collaboration among key sectors of society, including medical personnel, research workers, government, industry regulators, and the general public. While lessons learned from this endeavor should allow us to be better prepared for the next pandemic pathogen, a very large section of the world population, especially in the developing world, remain far from receiving the desired protection. Risks associated with emergence of newer variants, which might be more transmissible or even more virulent than the delta variant, loom large in the minds of clinicians and scientists who cannot be complacent. Viruses spreading rampantly would invariably mutate; whether such mutations would lead to a “gain of function” or a “loss of function” remains unpredictable. Development of more effective vaccines is the need of the day and the risks from vaccination, neurological or otherwise, would never match the havoc caused by the virus [114]. True, complications like CVST, ANE, GBS, and ADEM are potentially fatal ones; but almost all of these are treatable ones if detected early and therapeutic results are commonly predictable. This is very much unlike cases with severe COVID-19 infection. Furthermore, most severe neurological adverse events have been reported either in isolated case reports or small cases series. A causal association of these events is controversial; larger collaborative prospective studies are needed to establish causality. The overwhelming evidence as of now supports widescale vaccination as the risks of COVID-19 infection far outweigh those which may occur secondary to vaccination, and hence should not be the cause for vaccine hesitancy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization. Global vaccine action plan 2011–2020. WHO. https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ (2013). Accessed Oct 2022.
World Health Organization. Child mortality and causes of death. WHO. https://www.who.int/gho/child_health/mortality/mortality_under_five_text/en/ (2020). Accessed Oct 2022.
Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–6. https://doi.org/10.1126/science.abb8923.
Forni G, Mantovani A. COVID-19 Commission of Accademia Nazionale dei Lincei, Rome COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–39. https://doi.org/10.1038/s41418-020-00720-9.
Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. https://doi.org/10.1038/s41577-020-00479-7.
• Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. https://doi.org/10.1056/NEJMoa2034577. This article demonstrates the mechanism of action, safety and efficacy of the Pfizer-BioNTech vaccine against COVID19.
• Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2— preliminary report. N Engl J Med Overseas Ed. 2020;383:1920–31. https://doi.org/10.1056/NEJMoa2022483. This article discusses the mechanism of action and rate of adverse events of the Moderna vaccine against COVID19.
• Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–201. https://doi.org/10.1056/NEJMoa2101544. This article elaborates on the mechanism of action, efficacy and adverse events with the Ad26.COV2.S vaccine.
• Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–97. https://doi.org/10.1016/S0140-6736(20)31866-3. Article discussing the principle of the Sputnik V vaccine along with phase III trial data regarding efficacy and tolerability.
• Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. https://doi.org/10.1016/S0140-6736(20)32661-1. Article elucidating the phase II/III clinical trial data regarding vaccine efficacy and adverse event profile in the Oxford/ ChAdOx1 nCoV-19 vaccine.
• Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–83. https://doi.org/10.1056/NEJMoa2107659. Data from Phase III trial of Novavax including protection offered and adverse event profile.
• Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173–84. https://doi.org/10.1016/S0140-6736(21)02000-6. This article elucidates the mechanism of action, and reports the efficacy and safety data of phase III trial with Covaxin.
• Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021;111:219–26. https://doi.org/10.1016/j.ijid.2021.08.013. This article reviews the adverse events reported with the Sinopharm vaccine use in the UAE.
• Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–12. https://doi.org/10.1016/S1473-3099(20)30987-7. Article discussing potency and side effect profile of CoronaVac vaccine.
•• Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021;36(4):427–39. https://doi.org/10.1007/s12291-021-00968-z. This article provides a comprehensive review of the safety profile of the various licensed COVID19 vaccines.
Ganesan S, Al Ketbi LMB, Al Kaabi N, Al Mansoori M, Al Maskari NN, Al Shamsi MS, et al. Vaccine side effects following COVID-19 vaccination among the residents of the UAE-an observational study. Front Public Health. 2022;10:876336. https://doi.org/10.3389/fpubh.2022.876336.
• Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. BMJ. 2022;378:e069445. https://doi.org/10.1136/bmj-2021-069445. Article reviews available data regarding development of myocarditis and pericarditis post-COVID vaccination and possible mechanism.
•• Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3–40. https://doi.org/10.1007/s10072-021-05662-9. An exhaustive review of reported neurological adverse events with various COVID19 vaccines.
•• García-Grimshaw M, Ceballos-Liceaga SE, Hernández-Vanegas LE, Núñez I, Hernández-Valdivia N, Carrillo-García DA, et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: a nationwide descriptive study. Clin Immunol. 2021;229:108786. https://doi.org/10.1016/j.clim.2021.108786. The article reports on the incidence of neurological adverse events post-vaccination with the first dose of Pfizer-BioNTech vaccine in a Mexican cohort.
Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021;36(14):e107. https://doi.org/10.3346/jkms.2021.36.e107.
Lee YW, Lim SY, Lee JH, Lim JS, Kim M, Kwon S, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021;36(21):e153. https://doi.org/10.3346/jkms.2021.36.e153.
Göbel CH, Heinze A, Karstedt S, Morscheck M, Tashiro L, Cirkel A, et al. Headache attributed to vaccination against COVID-19 (coronavirus SARS-CoV-2) with the ChAdOx1 nCoV-19 (AZD1222) vaccine: a multicenter observational cohort study. Pain Ther. 2021;10(2):1309–30. https://doi.org/10.1007/s40122-021-00296-3.
• García-Azorín D, Do TP, Gantenbein AR, Hansen JM, Souza MNP, Obermann M, et al. Delayed headache after COVID-19 vaccination: a red flag for vaccine induced cerebral venous thrombosis. J Headache Pain. 2021;22(1):108. https://doi.org/10.1186/s10194-021-01324-5. This article provides strong evidence in favor of ruling out cerebral venous sinus thrombosis in patients with delayed onset headaches after adenoviral vaccines.
World Health Organization. COVID-19 vaccines: safety surveillance manual. World Health Organization. 2020. https://apps.who.int/iris/handle/10665/338400. Accessed Oct 2022.
•• Iba T, Levy JH, Warkentin TE. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2022;50(1):e80–6. https://doi.org/10.1097/CCM.0000000000005211. This article provides an extensive review of vaccine-induced thrombocytopenia following COVID19 vaccination.
•• Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2104840. The authors of this study demonstrated their patients to test positive on a screening platelet factor 4 (PF4)-heparin immunoassay although none of them had received heparin in the past. Patients also tested positive on a platelet-activation assay in the presence of PF4 independent of heparin. Platelet activation was inhibited by high levels of heparin and immunoglobulin . It was postulated that “the vaccine resulted in a rare thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, which clinically mimics autoimmune heparin-induced thrombocytopenia (HIT)”.
•• Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–11. https://doi.org/10.1056/NEJMoa2105385. Case series of patients who developed thrombosis with thrombocytopenia and positive antibodies to platelet factor 4 after inoculation with AstraZeneca vaccine, providing insights into mechanism and outcomes.
Bersinger S, Lagarde K, Marlu R, Pernod G, Payen JF. Using nonheparin anticoagulant to treat a near-fatal case with multiple venous thrombotic lesions during ChAdOx1 nCoV-19 vaccination-related vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021;49(9):e870–3. https://doi.org/10.1097/CCM.0000000000005105.
Graf T, Thiele T, Klingebiel R, Greinacher A, Schäbitz WR, Greeve I. Immediate high-dose intravenous immunoglobulins followed by direct thrombin-inhibitor treatment is crucial for survival in Sars-Covid-19-adenoviral vector vaccine-induced immune thrombotic thrombocytopenia VITT with cerebral sinus nvenous and portal vein thrombosis. J Neurol. 2021:20211–3. https://doi.org/10.1007/s00415-021-10599-2
George G, Friedman KD, Curtis BR, Lind SE. Successful treatment of thrombotic thrombocytopenia with cerebral sinus venous thrombosis following Ad26.COV2.S vaccination. Am J Hematol. 2021. https://doi.org/10.1002/ajh.26237.
Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138(4):350–3. https://doi.org/10.1182/blood.2021011958.
Schulz JB, Berlit P, Diener HC, Gerloff C, Greinacher A, Klein C, et al. COVID-19 Vaccine-Associated Cerebral Venous Thrombosis in Germany. Ann Neurol. 2021;90(4):627–39. https://doi.org/10.1002/ana.26172.
Gattringer T, Gressenberger P, Gary T, Wölfler A, Kneihsl M, Raggam RB. Successful management of vaccine-induced immune thrombotic thrombocytopenia-related cerebral sinus venous thrombosis after ChAdOx1 nCov-19 vaccination. Stroke Vasc Neurol.svn-2021–001142. 2021. https://doi.org/10.1136/svn-2021-001142
Clark RT, Johnson L, Billotti J, Foulds G, Ketels T, Heard K, et al. Early Outcomes of Bivalirudin Therapy for Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After Ad26.COV2.S Vaccination. Ann Emerg Med. 2021;78(4):511–4. https://doi.org/10.1016/j.annemergmed.2021.04.035.
•• Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. https://doi.org/10.1136/bmj.n1114. Study reporting arterial and hemostatic events after AstraZeneca vaccination in Denmark and Norway.
Krzywicka K, Heldner MR, Sánchez van Kammen M, van Haaps T, Hiltunen S, Silvis SM, et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021;28(11):3656–62. https://doi.org/10.1111/ene.15029.
McKeigue PM, Burgul R, Bishop J, Robertson C, McMenamin J, O’Leary M, et al. Association of cerebral venous thrombosis with recent COVID-19 vaccination: case-crossover study using ascertainment through neuroimaging in Scotland. BMC Infect Dis. 2021;21(1):1275. https://doi.org/10.1186/s12879-021-06960-5.
Perry RJ, Tamborska A, Singh B, Craven B, Marigold R, Arthur-Farraj P, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021;398(10306):1147–56. https://doi.org/10.1016/S0140-6736(21)01608-1.
• Chakravarty A. Between the devil and the deep blue sea? Case 3.14. In: Jaypee CA, editor. Neurology & Internal Medicine – A Case Based Study. New Delhi, London: Brothers Publications; 2021, p. 195–99. Report of a case of cerebral venous sinus thrombosis in a lady following Covisheild COVID 19 vaccination . The patient had an underlying Protein S deficiency and hence had been put on oral anticoagulation for indefinite period.
•• Covid-19 rapid guideline: Vaccine-induced immune thrombocytopenia and thrombosis (VITT) [Internet]. National Center for Biotechnology Information. U.S. National Library of Medicine; [cited 2022Nov6]. Available from: https://pubmed.ncbi.nlm.nih.gov/34406720/. Accessed Nov 2022. NICE guidelines for the diagnosis and management of VITT.
Bersinger S, Lagarde K, Marlu R, Pernod G, Payen JF. Using nonheparin anticoagulant to treat a near-fatal case with multiple venous thrombotic lesions during ChAdOx1 nCoV-19 vaccination-related vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021;49(9):e870–3. https://doi.org/10.1097/CCM.0000000000005105.
• Kakovan M, Ghorbani Shirkouhi S, Zarei M, Andalib S. Stroke Associated with COVID-19 Vaccines. J Stroke Cerebrovasc Dis. 2022;31(6):106440. https://doi.org/10.1016/j.jstrokecerebrovasdis.2022.106440. This article analyses data regarding strokes associated with COVID vaccination.
Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–5. https://doi.org/10.1111/jth.15347.
De Michele M, Iacobucci M, Chistolini A, Nicolini E, Pulcinelli F, Cerbelli B, et al. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat Commun. 2021;12(1):4663. https://doi.org/10.1038/s41467-021-25010-x.
Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;92(11):1247–8. https://doi.org/10.1136/jnnp-2021-326984.
• Suwanwela NC, Kijpaisalratana N, Tepmongkol S, et al. Prolonged migraine aura resembling ischemic stroke following CoronaVac vaccination: an extended case series. J Headache Pain. 2022;23(1):13. https://doi.org/10.1186/s10194-022-01385-0. Case series of a novel transient neurological phenomenon resembling prolonged migraine aura post administration of CoronaVac.
Bjørnstad-Tuveng TH, Rudjord A, Anker P. Fatal cerebral haemorrhage after COVID-19 vaccine. Fatal hjerneblødning etter covid-19-vaksine. Tidsskr Nor Laegeforen. 2021;141. https://doi.org/10.4045/tidsskr.21.0312
de Melo Silva ML, Lopes DP. Large hemorrhagic stroke after ChAdOx1 nCoV-19 vaccination: a case report. Acta Neurol Scand. 2021. https://doi.org/10.1111/ane.13505.
Finsterer J, Korn M. Aphasia seven days after second dose of an mRNA-based SARS-CoV-2 vaccine. Brain Hemorrhages. 2021;2:165–7. https://doi.org/10.1016/j.hest.2021.06.001.
Robichaud J, Côté C, Côté F. Systemic capillary leak syndrome after ChAdOx1 nCOV-19 (Oxford-AstraZeneca) vaccination. CMAJ. 2021;193(34):E1341–4. https://doi.org/10.1503/cmaj.211212.
Rosso M, Anziska Y, Levine SR. Acute transient encephalopathy after Moderna COVID-19 vaccine. Case Rep Neurol. 2022;14(2):231–6. https://doi.org/10.1159/000523769.PMID:35702446;PMCID:PMC9149540.
• Liu BD, Ugolini C, Jha P. Two cases of post-Moderna COVID-19 vaccine encephalopathy associated with nonconvulsive status epilepticus. Cureus. 2021;13(7):e16172. https://doi.org/10.7759/cureus.16172. Case reports of new onset encephalopathy with non-convulsive status epilepticus following administration of the Moderna vaccine.
Al-Mashdali AF, Ata YM, Sadik N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: a case report. Ann Med Surg (Lond). 2021;69:102803. https://doi.org/10.1016/j.amsu.2021.102803.
•• Permezel F, Borojevic B, Lau S, de Boer HH. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci Med Pathol. 2022;18(1):74–9. https://doi.org/10.1007/s12024-021-00440-7. This article summarizes the clinical features and autopsy findings of the first fatal case of ADEM following Oxford/AstraZeneca vaccine.
OzgenKenangil G, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine [published correction appears in Acta Neurol Belg. Acta Neurol Belg. 2021;121(4):1089–91. https://doi.org/10.1007/s13760-021-01699-x.
Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2022;122(3):793–5. https://doi.org/10.1007/s13760-021-01608-2.
•• Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal Encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90(3):506–11. https://doi.org/10.1002/ana.26182. Case series of 23 patients with post-vaccinal encephalitis fulfilling the criteria for autoimmune encephalitis after vaccination with the Oxford/AstraZeneca vaccine.
Kobayashi Y, Karasawa S, Ohashi N, Yamamoto K. A case of encephalitis following COVID-19 vaccine. J Infect Chemother. 2022;28(7):975–7. https://doi.org/10.1016/j.jiac.2022.02.009.
Bensaidane MR, Picher-Martel V, Émond F, De Serres G, Dupré N, Beauchemin P. Case report: Acute necrotizing encephalopathy following COVID-19 vaccine. Front Neurol. 2022;13:872734. https://doi.org/10.3389/fneur.2022.872734.
Malhotra SH, Gupta P, Prabhu V, Garg RK, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis. QJM. 2021;hcab069. https://doi.org/10.1093/qjmed/hcab069
Tan WY, Yusof Khan AHK, MohdYaakob MN, Abdul Rashid AM, Loh WC, Baharin J, et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: a case report. BMC Neurol. 2021;21(1):395. https://doi.org/10.1186/s12883-021-02427-x.
Pagenkopf C, Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol. 2021;358:577606. https://doi.org/10.1016/j.jneuroim.2021.577606.
Chagla Z. In adults, the Oxford/AstraZeneca vaccine had 70% efficacy against COVID-19 >14 d after the 2nd dose. Ann Intern Med. 2021;174(3):JC29. https://doi.org/10.7326/ACPJ202103160-029.
Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–4. https://doi.org/10.1016/S0140-6736(20)32623-4.
Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222). Front Immunol. 2021;26(12):653786. https://doi.org/10.3389/fimmu.2021.653786.PMID:33981305;PMCID:PMC8107358.
• Quintanilla-Bordás C, Gascón-Gimenez F, Alcalá C, Payá M, Mallada J, Silla R, et al. Case report: Exacerbation of relapses following mRNA COVID-19 vaccination in multiple sclerosis: a case series. Front Neurol. 2022;13:897275. https://doi.org/10.3389/fneur.2022.897275. Case series of patients with yet-unrecognized multiple sclerosis whose relapses flared with mRNA vaccines and possible mechanisms and immune markers.
•• Epstein S, Xia Z, Lee AJ, Dahl M, Edwards K, Levit E, et al. Vaccination against SARS-CoV-2 in neuroinflammatory disease: early safety/tolerability data. Mult Scler Relat Disord. 2022;57:103433. https://doi.org/10.1016/j.msard.2021.103433. A large cross-sectional study demonstrating the safety of vaccination in patients with neuroimmunological disorders.
Dinoto A, Gastaldi M, Iorio R, Marini S, Damato V, Farina A, et al. Safety profile of SARS-CoV-2 vaccination in patients with antibody-mediated CNS disorders. Mult Scler Relat Disord. 2022;63:103827. https://doi.org/10.1016/j.msard.2022.103827.
Alfishawy M, Bitar Z, Elgazzar A, Elzoueiry M. Neuroleptic malignant syndrome following COVID-19 vaccination. Am J Emerg Med. 2021;49:408–9. https://doi.org/10.1016/j.ajem.2021.02.011.
Nagamine T. Neuroleptic malignant syndrome associated with COVID-19 vaccination. CJEM. 2022;24(3):349–50. https://doi.org/10.1007/s43678-021-00254-0.
Ghosh R, Dubey S, Roy D, Mandal A, Naga D, Benito-León J. Focal onset non-motor seizure following COVID-19 vaccination: a mere coincidence? Diabetes Metab Syndr. 2021;15(3):1023–4. https://doi.org/10.1016/j.dsx.2021.05.003.
Makhlouf AT, Van Alphen MU, Manzano GS, Freudenreich O. A seizure after COVID-19 vaccination in a patient on clozapine. J Clin Psychopharmacol. 2021;41(6):689–90. https://doi.org/10.1097/JCP.0000000000001488.
• Werner J, Brandi G, Jelcic I, Galovic M. New-onset refractory status epilepticus due to autoimmune encephalitis after vaccination against SARS-CoV-2: first case report. Front Neurol. 2022;13:946644. https://doi.org/10.3389/fneur.2022.946644. Case report of new-onset refractory status epilepticus with seronegative autoimmune encephalitis after Pfizer/BioNTech vaccine.
•• Martinez-Fernandez I, Sanchez-Larsen A, Gonzalez-Villar E, Martínez-Martín Á, von Quednow E, Del Valle-Pérez JA, et al. Observational retrospective analysis of vaccination against SARS-CoV-2 and seizures: VACCI-COVID registry. Epilepsy Behav. 2022;134:108808. https://doi.org/10.1016/j.yebeh.2022.10880. Retrospective study demonstrating the overall safety of COVID vaccines in patients with epilepsy.
• Özdemir HN, Dere B, Gökçay F, Gökçay A. Are COVID-19 vaccines safe for people with epilepsy? A cross-sectional study Neurol Sci. 2022;43(6):3489–96. https://doi.org/10.1007/s10072-022-05956-6. Cross-sectional study showing that COVID vaccines are well tolerated in patients with epilepsy.
von Wrede R, Pukropski J, Moskau-Hartmann S, Surges R, Baumgartner T. COVID-19 vaccination in patients with epilepsy: First experiences in a German tertiary epilepsy center. Epilepsy Behav. 2021;122:108160. https://doi.org/10.1016/j.yebeh.2021.108160.
Massoud F, Ahmad SF, Hassan AM, Alexander KJ, Al-Hashel J, Arabi M. Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): a cross-sectional study. Seizure. 2021;92:2–9. https://doi.org/10.1016/j.seizure.2021.08.001.
Li N, Chu C, Lin W. A survey of hesitancy and response to the COVID-19 vaccine among patients with epilepsy in northeast China. Front Neurol. 2021;2:778618. https://doi.org/10.3389/fneur.2021.778618.
• Clayton LM, Balestrini S, Cross JH, Wilson G, Eldred C, Evans H, et al. The impact of SARS-COV-2 vaccination in Dravet syndrome: a UK survey. Epilepsy Behav. 2021;124:108258. https://doi.org/10.1016/j.yebeh.2021.108258. This article provides data supporting the safety of COVID vaccination in patients with Dravet Syndrome.
Lu Q, Wang YY, Wang QH, Tang LN, Yang XY, Dun S, et al. Safety of inactivated COVID-19 vaccine in tuberous sclerosis complex patients with epilepsy treated with rapamycin. Seizure. 2022;99:71–4. https://doi.org/10.1016/j.seizure.2022.05.010.
Shemer A, Pras E, Hecht I. Peripheral facial nerve palsy following BNT162b2 (COVID-19) vaccination. Isr Med Assoc J. 2021;23(3):143–4 (PMID: 33734623).
Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14(7):e243829. https://doi.org/10.1136/bcr-2021-243829.
Obermann M, Krasniqi M, Ewers N, Fayad J, Haeberle U. Bell’s palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci. 2021;42(11):4397–9. https://doi.org/10.1007/s10072-021-05496-5.
Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021;147(8):739–43. https://doi.org/10.1001/jamaoto.2021.1259.
•• Ozonoff A, Nanishi E, Levy O. Bell’s palsy and SARSCoV-2 vaccines. Lancet Infect Dis. 2021;21(4):450–2. https://doi.org/10.1016/S1473-3099(21)00076-1. This article reviews data regarding Bell’s Palsy post-COVID vaccination demonstrating the increased risk of the same.
Lechien JR, Diallo AO, Dachy B, Le Bon SD, Maniaci A, Vaira LA, Saussez S (2021) COVID-19: Post-vaccine smell and taste disorders: report of 6 cases. Ear Nose Throat J Sep 1:1455613211033125. https://doi.org/10.1177/01455613211033125. Epub ahead of print. PMID: 34467793.
Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133–7. https://doi.org/10.1097/RMR.0000000000000287.
Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J AAPOS. 2021;25(5):302–3. https://doi.org/10.1016/j.jaapos.2021.05.003.
Sen M, Honavar SG. After the storm: ophthalmic manifestations of COVID-19 vaccines. Indian J Ophthalmol. 2021;69(12):3398–420. https://doi.org/10.4103/ijo.IJO_2824_21.
Chuang TY, Burda K, Teklemariam E, Athar K. Tolosa-Hunt syndrome presenting after COVID-19 vaccination. Cureus. 2021;13(7):e16791. https://doi.org/10.7759/cureus.16791.
Bialasiewicz AA, Farah-Diab MS, Mebarki HT. Central retinal vein occlusion occurring immediately after 2nd dose of mRNA SARS-CoV-2 vaccine. Int Ophthalmol. 2021;41:3889–92. https://doi.org/10.1007/s10792-021-01971-2.
•• Alvarez LM, NingNeo Y, Davagnanam I, Ashenhurst M, Acheson J, Abdel-Hay A, et al. Post vaccination optic neuritis: Observations from the SARS-CoV-2 pandemic. SSRN Electron J. 2021. https://doi.org/10.2139/ssrn.3889990. Review article studying risk of optic neuritis post-vaccination, concluding that no definite increase in rates of optic neuritis after inoculation.
Formeister EJ, Wu MJ, Chari DA, Meek R 3rd, Rauch SD, Remenschneider AK, et al. Assessment of sudden sensorineural hearing loss after COVID-19 vaccination. JAMA Otolaryngol Head Neck Surg. 2022;148(4):307–15. https://doi.org/10.1001/jamaoto.2021.4414.
Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological Complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13(2):e13426. https://doi.org/10.7759/cureus.13426.
• Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology. 2021. https://doi.org/10.1212/WNL.0000000000011881. Study of risk of GBS post-COVID vaccination demonstrating the absence of sufficient evidence to implicate vaccines as the cause.
Kohli S, Varshney M, Mangla S, Jaiswal B, Chhabra PH. Guillain-Barré syndrome after COVID-19 vaccine: should we assume a causal link? International Journal of Medical and Pharmaceutical Case Reports. 2021;20–4. https://doi.org/10.9734/ijmpcr/2021/v14i130124.
Bonifacio GB, Patel D, Cook S, Purcaru E, Couzins M, Domjan J, et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry. 2021;93(3):341–2. https://doi.org/10.1136/jnnp-2021-327027.
McKean N, Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14(7):e244125. https://doi.org/10.1136/bcr-2021-244125.
Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64(1):E1–2. https://doi.org/10.1002/mus.27251.
Mahajan S, Zhang F, Mahajan A, Zimnowodzki S. Parsonage Turner syndrome after COVID-19 vaccination. Muscle Nerve. 2021;64(1):E3–4. https://doi.org/10.1002/mus.27255.
McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. https://doi.org/10.1016/j.jaad.2021.03.092.
• Nassar M, Chung H, Dhayaparan Y, Nyein A, Acevedo BJ, Chicos C, Zheng D, Barras M, Mohamed M, Alfishawy M, Nso N, Rizzo V, Kimball E. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab Syndr. 2021;15(4):102170. https://doi.org/10.1016/j.dsx.2021.06.007. Case report of possible vaccine-induced rhabdomyolysis with good outcome following prompt treatment.
Kimura M, Niwa JI, Doyu M. Recurring weakness in rhabdomyolysis following Pfizer-BioNTech coronavirus disease 2019 mRNA vaccination. Vaccines (Basel). 2022;10(6):935. https://doi.org/10.3390/vaccines10060935.
•• Sansone G, Bonifati DM. Vaccines and myasthenia gravis: a comprehensive review and retrospective study of SARS-CoV-2 vaccination in a large cohort of myasthenic patients. J Neurol. 2022;269:3965–81. https://doi.org/10.1007/s00415-022-11140-9. This review article examines the available data regarding safety of COVID vaccines in myasthenia patients.
Cosentino C, Torres L, Vélez M, et al. SARS-CoV-2 vaccines and motor symptoms in Parkinson’s disease. Mov Disord. 2022;37(1):233. https://doi.org/10.1002/mds.28851.
Erro R, Buonomo AR, Barone P, Pellecchia MT. Severe dyskinesia after administration of SARS-CoV2 mRNA vaccine in Parkinson’s disease. Mov Disord. 2021;36(10):2219. https://doi.org/10.1002/mds.28772.
•• Imbalzano G, Ledda C, Artusi CA, Romagnolo A, Montanaro E, Rizzone MG, et al. SARS-CoV-2 vaccination, Parkinson’s disease, and other movement disorders: case series and short literature review. Neurol Sci. 2022;43:5165–8. https://doi.org/10.1007/s10072-022-06182-w. Case report and review of other cases of Parkinsonism showing transient worsening of motor symptoms following COVID vaccination.
• Naharci MI, Tasci I. Delirium in a patient with Alzheimer’s dementia following COVID-19 vaccination. Psychogeriatrics. 2021;21(5):846–7. https://doi.org/10.1111/psyg.12747. Case report of precipitation of delirium in a patient with Alzheimer’s disease after CoronaVac Vaccine.
Ng JH, Chaudhuri KR, Tan EK. Functional neurological disorders and COVID-19 vaccination. Ann Neurol. 2021;90(2):328. https://doi.org/10.1002/ana.26160.
Takahashi O, Sakakibara R, Sawai S, Ogata T. Functional neurological disorders after COVID-19 vaccination: Case series and literature review. Psychiatry Clin Neurosci. 2022;25. https://doi.org/10.1111/pcn.13453.
Kim DD, Kung CS, Perez DL. Helping the public understand adverse events associated with covid-19 vaccinations. JAMA Neurol. 2021;78(7):789. https://doi.org/10.1001/jamaneurol.2021.1042.
•• Butler M, Coebergh J, Safavi F, Carson A, Hallett M, Michael B, et al. 2021 Functional neurological disorder after SARS-CoV-2 vaccines: two case reports and discussion of potential public health implications. J Neuropsychiatry Clin Neurosci. https://doi.org/10.1176/appi.neuropsych.21050116. Reports of patients developing functional neurological disorder following COVID-vaccination and implications.
Ercoli T, Lutzoni L, Orofino G, Muroni A, Defazio G. Functional neurological disorder after COVID-19 vaccination. Neurol Sci. 2021;42:1–2. https://doi.org/10.1007/s10072-021-05504-8.
Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856–7. https://doi.org/10.1002/ana.26065.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Aparajita Chatterjee and Ambar Chakravarty each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of Topical Collection on Neurology of Systemic Diseases
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chatterjee, A., Chakravarty, A. Neurological Complications Following COVID-19 Vaccination. Curr Neurol Neurosci Rep 23, 1–14 (2023). https://doi.org/10.1007/s11910-022-01247-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01247-x