EVIDENCE SUMMARY
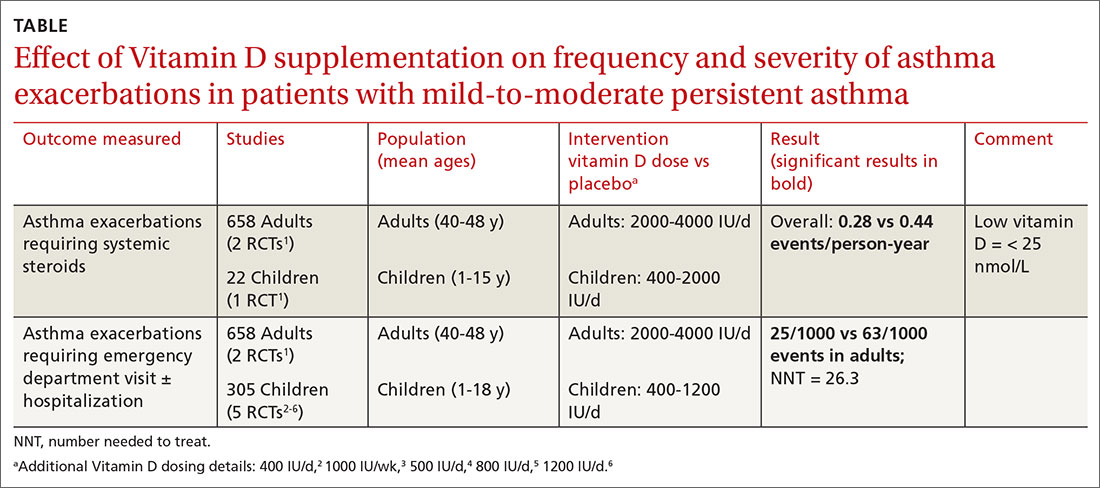
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS