See how surgeons replace a faulty heart valve
Under the Knife: When patients suffer from aortic stenosis their heart valves do not fully open. To treat this, doctors often recommend surgery to implant a prosthetic valve. Dr. Manny goes into the operating room to show us exactly how it’s done
Patients who suffer from aortic stenosis have heart valves that do not fully open. The condition decreases blood flow from the heart, so doctors often recommend surgery to implant a prosthetic valve.
CoreValve, a new minimally invasive system to replace faulty heart valves, was approved by the U.S. Food and Drug Administration in January 2014.
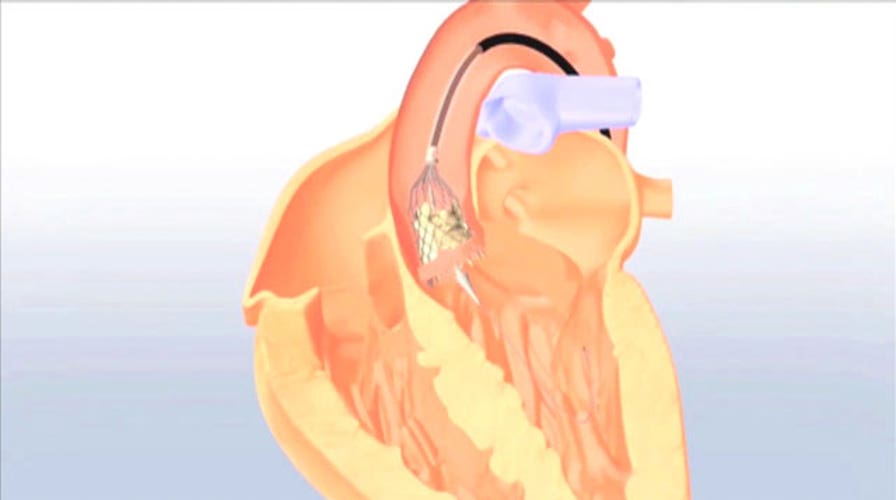
During the procedure, the physician makes a small incision into a patient’s femoral artery, located in the groin. They then insert the CoreValve device with a catheter, guiding it through the arteries to the site of the narrowed aortic valve in the heart.
The design of the artificial valve makes it possible to treat patients with a vascular system that is small or difficult to navigate.
Once in place, the CoreValve's self-expanding frame enables physicians to deploy it in a controlled manner, allowing for accurate placement.
The device expands and takes over the original valve's function to ensure that oxygen-rich blood flows efficiently out of the heart and circulates throughout the rest of the body.
The catheter is removed and the incision in the groin is closed.
After surgery, the doctor will schedule regular follow-up appointments to check the valve.
Click here for more information on CoreValve.